


![]()
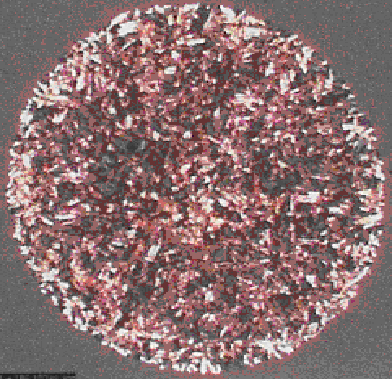
MALDI Old vs New, PMMA ca. 100 nLs (USF Data) and 5x40 nL IBF drops.
Is The Dried Droplet “Method” Science?
In the earliest days of hyphenated mass spectrometry techniques, there existed multiple problems that made “quantitative” MS applications almost impossible. Problems from slow data scan speeds and data acquisition rates, along with vacuum system and interface problems, to poor chromatography and poor signal processing with minimal post data acquisition analytical data processing often resulted in many hyphenated MS techniques being referred to as “semi-quantitative,” not unlike MALDI is often referred to today.
Since quantitative and qualitative MS are not different entities and are, in fact, different parts of the same equation (1,2,3,4,5,6,7,8,9,10), such a statement is formally incorrect. When one acquires a mass spectrum, one always does both qualitative (m/z values) and quantitative (intensity values) analysis, no matter what one calls it. So the term “semi quantitative” has always meant to me that analytical problems cause the data to not be trusted as it relates the intensity axis. Now, it doesn’t take much to realize that with such a caveat on any data set, the qualitative “certainty” can be subject to doubt as well. (How do you know any m/z values is or is not zero? Answer: you don’t.). That stated I confess that I have done semi-quantitative analysis too.
As it happened new technology has in every aspect of hyphenated MS techniques over come the problems with early MS and MS/MS techniques. Technologies from fused silica GC columns to new LC columns, to better vacuum systems, ion optics and interface systems, to faster processors and powerful acquisition and post acquisition software along with new detectors allowed hyphenated MS techniques to become exquisitely quantitative, as we all know.
That stated, for the last twenty years, ESI and MALDI have been at the forefront of many MS research labs. Just as early GC/MS and LC/M S and MS/MS analysis variants had problems being quantitative, early ESI and MALDI had problems as well. To this day, while both techniques can be quantitative, it can still be difficult and MALDI can have particular problems.
In particular, MALDI ionization is an extremely complex process. Also, to this day many often use relatively primitive sample deposition devices to dispense small quantities (2.0 to 0.5 uLs) of matrix/sample onto a target. Quite naturally this simple crude practice leads to very significant volumetric errors with the volatile solvents and additives employed. In the past, I assert that no one, knowing anything about analytical chemistry would have ever proposed such a transfer technique, let alone try to publish it. I am referring to the dried droplet “method.”
In this applied MS deposition technique, after the imprecise application of the volatile liquid of matrix, additives and analyte to the target, it is common knowledge that it often “crystallizes” into an irregularly shaped, heterogeneous mass (or mess) for subsequent analysis by million dollar instruments. It is also common knowledge that the MALDI ionization process is extremely complex and considering the fact that there is no ion containment after a laser shot onto heterogeneous samples that are of different morphology from spot to spot. Obviously, the intensity axis for any absolute or relative position on a MALDI plate, is simply not reproducible. As such, the sample preparation step in MALDI is completely out of control. As those that use MS and MS/MS learned many years, if d(n)/d(t) into the ion source is not reproducible, as use to be the case in GC/MS and LC/MS and MS/MS variants..... everything that follows won’t be reproducible as well, obviously.
Many others have sought to address the sample preparation issue including the use of ionic liquids championed by M. L. Gross (11) to acquire sample homogeneity. Others have used non-solvent sample prep methods and others have applied many other derivatives to improve MALDI sample preparation. To further improve MALDI and for other many other applications, we have invented and patented induction based fluidics, a technique that allows for the accurate, non-touch placement of nanoliter and microliter quantities of liquids well within 5 to 10 percent at one SD across most of that volume range across many different types of liquids. So good volumetric control can be obtained with our nanoliter devices. That is step one as it relates to good analytical chemistry. (12,13,14 and 15).
Then as it turns out we also get great sample morphology and a number of groups using our technique and devices have observed that with nanoliter dispensing the signal/noise ratio greatly improves (12,14,15). We have shown that IBF yields excellent sample morphology with S/N increases typically from a factor of 5 to 10 even up to a factor of 100 for proteins, peptides and synthetic polymers.....even without optimization studies. While the improvement is, of course, analyte, technique and system dependent, virtually everyone that has used the technique to date has seen major MALDI improvements in BOTH precision and senstivity whether it is positive ion reflectron mode with ionic liquid matrices for proteins or negative ion linear mode with solid matrices for peptides & proteins or even for positive ion mode reflectron mode for synthetic peptides with Mx's exceeding 100,000 daltons.
These observations and common analytical sense lead us to conclude that, in fact, for too many reasons to list, the dried droplet method is NOT a "method" and it is in fact NOT SCIENCE, no matter how many times it is published in peer review scientific literature. It is, in fact, analytically ludicrous to attempt to accurately place low uL quantities of volatile liquids using primitive dispensing tools which generating irregularly shaped, heterogeneous crystals and then to expect good MS results. Yet it is done all of the time, even by those studying fundamentals!
In a disclosure statement, it is true that Nanoliter LLC has a vested interest in developing and selling nL-IBF technology that can remedy this problem and which has many other applications. That stated, under any circumstances, it should be technically obvious or should I say that it used to be technically obvious that trying to places low uL quantities of volatile liquids onto plates accurately with primitive syringes which generate irregularly shaped heterogeneous spots which are then blasted by a laser, without ion containment and often without an internal standard and most often without a calibration ration curve, is not analytical chemistry and it is not therefore science.
In closing we would note that at ASMS (13) we not only showed some of the above but we demonstrated that with IBF, such sample preparation can be realized on a 1z-2z basis using low cost nanoliter syringes, or that one can on a robotic platform shoot liquids from capillaries, SPE devices and LC columns using our patented and patent pending technology for very rapid, accurate sample placement. This approach yields excellent morphology and analytical results for proteins, peptides and synthetic polymers in millisecond timeframes on a per liquid shot basis via capillaries, SPE or LC columns..
So now, there is no reason to use primitive dispensing tools that will call serious questions on your research or project. In fact, we believe that in the very near term, in at least limited objective scenarios that MALDI will become a routinely quantitative MS technique using IBF technology. IBF technologies can 1. dispense or 2. dispense & treat liquids that it 3. launches and it 4. directs liquids to targets in a 5. non-dispersive manner, that can be 6. measured or counted with the same circuit N, channels at a time across the widest dynamic range (uL to pL) at the lowest cost per channel across of any technology, optionally using disposables and using no moving parts.
References
1. A.D. Sauter, L.D. Betowski, B.N. Colby, T.R. Smith, and R.G. Beimer, “Fused Silica Capillary Column GC/MS Analysis for Priority Pollutants,” Journal of High Resolution Chromatography and Chromatography Communications, August, 1981, 366-384.
2. A.D. Sauter, P.M. Mills, W.L. Fitch, and V. Lopez-Avila, “Inter-laboratory GC/MS Response Factor Precision,” Journal of High Resolution Chromatography and Chromatography Communications, January, 1982, 27-30.
3. W.L. Fitch and A.D. Sauter, “Calculation of Relative Electron Impact Total Ionization Cross Sections for Organic Molecules,” Anal. Chem. 1983, 55, 832
4. A.D. Sauter, L.D. Betowski, and J.M. Ballard, “A Comparison of Response Factors for Triple and Single Stage Quadrupole Mass Spectrometers,” Anal. Chem. 1983, 55,116.
5. L.D. Betowski, H. Webb, and A.D. Sauter, “The Application of Pulsed Positive Ion Negative Ion Chemical Ionization”, Biomedical Mass Spectrometry, 1983, Vol. 10, No. 6, 369.
6. A.D. Sauter and J.J. Downs et al, “Model for the Estimation of Electron Impact GC/MS Response Factors for Quadrupole Mass Spectrometers,” Analytical. Chem. 1986, 58, 1665.
7. A. D. Sauter, W. Fitch, J. Chakel, A. Affel, R. Willoughby and E. Sheenan, "Approaches For Estimating ESI/MS/MS Relative Response,”Pittcon 97, New Orleans, LA, March 1997.
8. A. D. Sauter, USA Patent Application resulting in US Patent No. 6,149,815, November, 1999 awarded November, 20,2000. US pending patents of A.D. Sauter: 60/574,104; 60/759,787;60/881,532 and 61/011,178 and others.
9. A. D. Sauter and A. D. Sauter III, “Nanoliters Onto Media, Use Of Electric Induction, American Laboratory, October, 2001.
10. A. D. Sauter and A. D. Sauter III, “ ‘Electric’ Zip Tips For MALDI And Other Applications,” Journal of the Association of Laboratory Automation, May-June 2002.
11. Ying L. Li and Michael L. Gross, “Ionic Liquid Matrices for Quantitative Analysis by MALDI TOF Mass Spectroscopy”, JASMS, 2004,15,1833-1837.
12. T. Tu, A. D. Sauter Jr., A. D. Sauter III and M.L. Gross, Improving Intensity and Sensitivity of MALDI Signals by Nanoliter Volume Spotting, JASMS, in press. Sept 2008.
13. A. D. Sauter, et al, “Millisecond Nanoliters,” presented at the 56th ASMS Conference on Mass Spectroscopy, Denver CO, June 2008.
14. B. Hilker, K. J. Clifford, A. D. Sauter Jr, A. D. Sauter III, and Julie P. Harmon,. Measuring Charge For The Real Time Induction Based Fluidic MALDI Dispense Event Verification and Nanoliter Volume Determination accepted with revision JASMS,12/ 2008.
15. Hilker, B.; Clifford, K. J.; Sauter Jr., A.D.; Sauter III, A.D.; Gauthier, T.; Harmon, J.P. Electric Field Enhanced Sample Preparation for Synthetic Polymer MALDI-TOF Mass Spectrometry via Induction Based Fluidics (IBF). Accepted with revision, Polymer, 12/2008.